| Classification |
Retinal Pigment Epithelial Cells (hES-RPE) |
| Indication |
Stargardt’s Macular Dystrophy (SMD) |
Age-related Macular Degeneration (AMD) |
Myopic Macular Degeneration (MMD) |
| Significance |
- World's first commercialization of embryonic stem cell therapy
- Fundamental treatment for Macular Degeneration |
| Characteristics |
- Leads to gene mutation cause vision loss |
- Affects older adults and results in loss of vision due to retinal damage
- Increases patients as population aging |
- Occurs retinal abnormalities in serious short-sightedness |
| Progress |
- 2009 : Partnered with ACT (U.S) to introduce pipeline
- 2010 : Received an orphan drug designation from U.S. FDA
(Benefits include 7-year marketing exclusivity, tax credits, grants for clinical development,
accelerated FDA approval, drug approval) |
- Clinical trial approval from U.S.FDA (2010.11)
- Clinical trial approval from Korea MFDS (2011.05) |
- Clinical trial approval from U.S. FDA (2011.01)
- Clinical trial approval from Korea MFDS (2012.05) |
- Clinical trial approval from U.S. FDA (2013.02)
- Clinical trial approval from Korea MFDS (2013.10) |
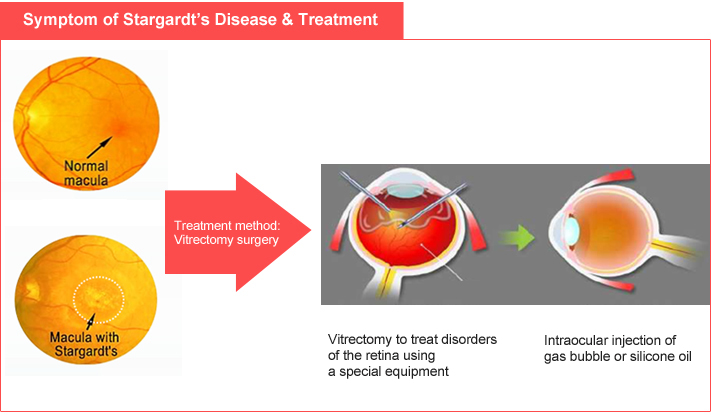
| Procedure |
- Step 1 : Vitrectomy surgery
- Step 2 : Subretinal transplantation of human embryonic stem cell-derived retinal pigment epithelial cells |
| Outcome |
- No safety issues related to the transplanted cells
- Improved ETDRS visual acuity among subjects
- Korea Study: 9-19 letters improvement in 3 patients from 4 patients analysis
- U.S. and Europe Study: >15 letters improvement in 6 patients from 18 patients analysis |
- Clinical trial in progress |